Видео ютуба по тегу Understanding Electron Configuration: A Guide For Beginners

(a) As described in Section 7.7 , the alkali metals react with hydrogen to form hydrides and react …

Consider element 113, Nh. What is the expected electron configuration for Nh? What oxidation states…

Identify the following elements based on their electron configurations and rank them in order of in…

In the experiment shown schematically below, a beam of neutral atoms is passed through a magnetic f…
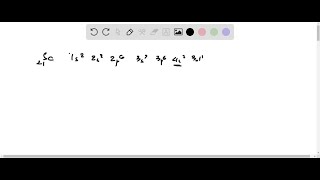
What do you suppose are the electron configuration and the formula of the monatomic ion formed by s…

Some ions do not have a corresponding neutral atom that has the same electron configuration. For ea…

Write an electron configuration for Ne. Then write a Lewis symbol for Ne and show which electrons f…

(a) Write the electron configuration for Li and estimate the efective nuclear charge experienced by…

Write the electron configuration for N. Then write the Lewis symbol for N and show which electrons …

Write ground-state electron configurations for the following elements. a. bromine (Br) C. antimony …
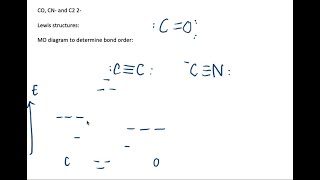
The species CO, CN^-, and C_2^2- are isoelectronic. (a) Draw their…

When a transition metal atom forms an ion, which electrons are lost first?

Explain bricfly why each of the following is not a possible set of quantum numbers for an electron …

Give three examples of ions that have an electron configuration of n d^8(n=3,4,5, …).

Considering only the ground-state electron configuration, are there more diamagnetic or paramagneti…

Write a Lewis symbol for each atom or ion. a. S2- b. Mg c. Mg2+ d. P

Write the full electron configuration (1 s^2 2 s^2, etc. ) for each of the …

Electron Configuration The Ultimate Guide for Beginners by The Science Starter Kit

Answer the following questions based on the given electron configurations, and identify the element…

State the periodic law, and explain its relation to electron configuration. (Use Na and K in your e…